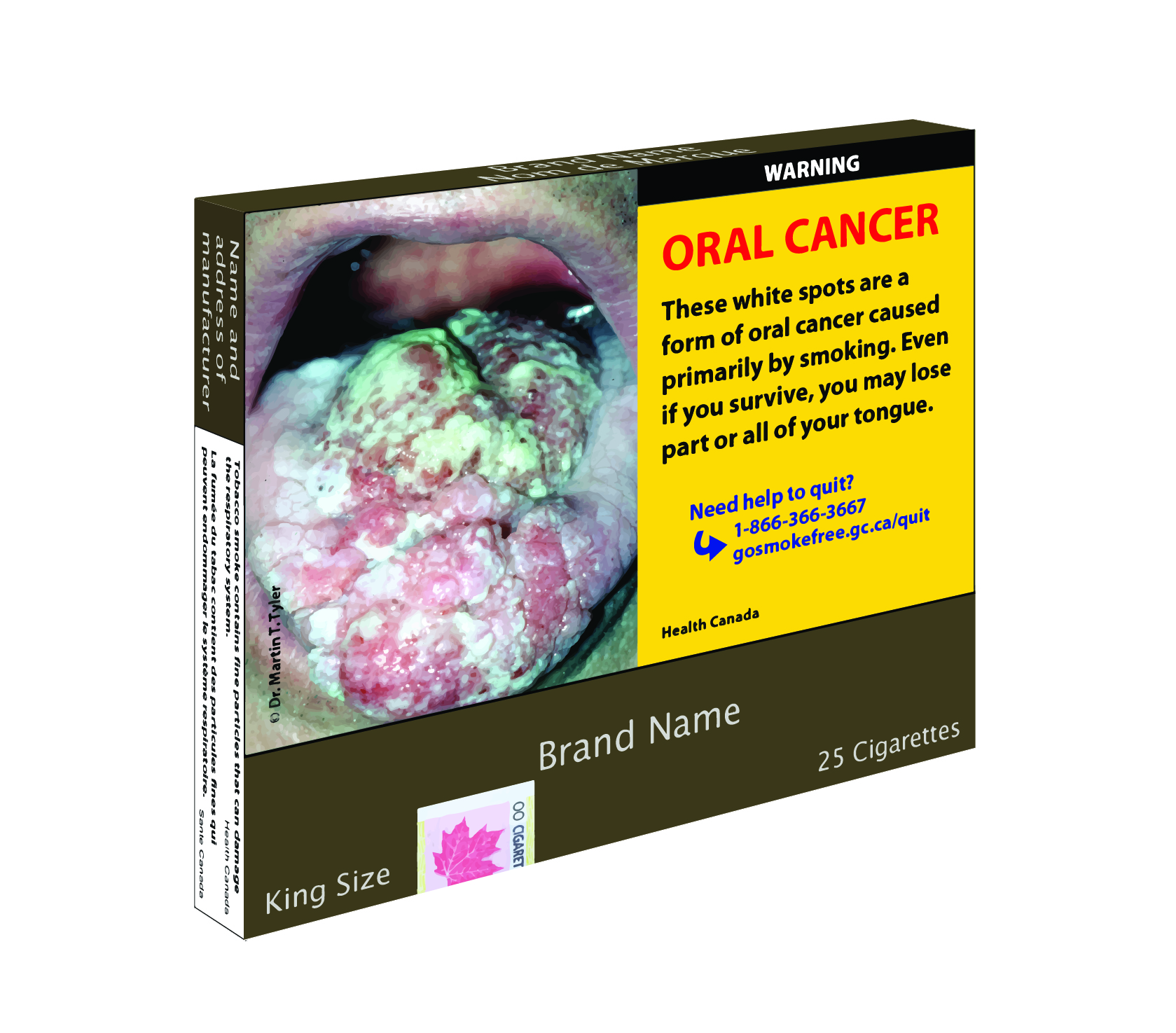
The Canadian Parliament has passed legislation that is a major overhaul to the country’s Tobacco Act. Yesterday the Parliament passed Bill S-5, which amends Canada’s current Tobacco Act and gives Health Canada the authority to implement plain packaging on tobacco products – including premium cigars.
The Parliament vote comes less than 24 hours from when a United States Federal Court upheld a challenge by the U.S. cigar industry contesting the U.S. Food and Drug Administration (FDA)’s Warning Label requirements. For the Canadian tobacco market, things are a lot worse.
For all practical purposes, Health Canada is Canada’s equivalent of the FDA in the U.S. They are responsible for compliance and enforcement of regulations pertaining to public health. While Parliament has not mandated how Health Canada should implement plain packaging, the Canadian department has been circulating its plans for the past couple of years. Essentially the law states packaging must contain prescribed information, including health hazards. The Canadian plan is expected to be based on Australia’s plain packaging law – considered to be the most austere in the world.
The new laws apply to all tobacco products: cigarettes, cigars, pipe tobacco, smokeless tobacco as well as vaping products.
Bill S-5 also introduces sweeping regulations that will now be imposed on the Canadian vaping industry that go well beyond packaging.
The timetable for implementation has not been disclosed yet, but it could begin as early as 180 days from yesterday.
Photo Credits: Health Canada







May 17, 2018 @ 11:16 am
Nanny-state. Communists love social engineering and fear mongering . . . meanwhile soon it’ll be “toke til’ you choke on weed” in Canada; however, no one seems to mention all of the risks when it comes to combusting cannabis, because “weed never hurt anyone bro, it’s totally natural”.
May 17, 2018 @ 11:30 am
. . . forgot, Cigar smokers can pretty much forget about new offerings and brands, apart from those already on the shelf since no company in their right mind would bother trying to market anything under such stifling requirements. In fact a few may pull-out and leave for good, Canada is a micro-market to begin with.
May 19, 2018 @ 3:10 pm
This piece of idiotic legislation will pave the way for counterfeiters to have a field day in the premium cigar market. Weed has been legalized yet warning labels on tobacco products are not enough. I understand that cigarettes cause a high incidence of cancer but to lump premium cigars, which are not inhaled, and are smoked far less frequently by aficionados is a cruel joke. In the end it will be small family owned retailers that will be hurt by this legislation.
The Scoop With Coop – KMA Talk Radio
June 25, 2018 @ 12:41 pm
[…] Canadian Parliament Passes Bill Paving Way for Plain Packaging on Tobacco […]